No products in the cart.
Articles
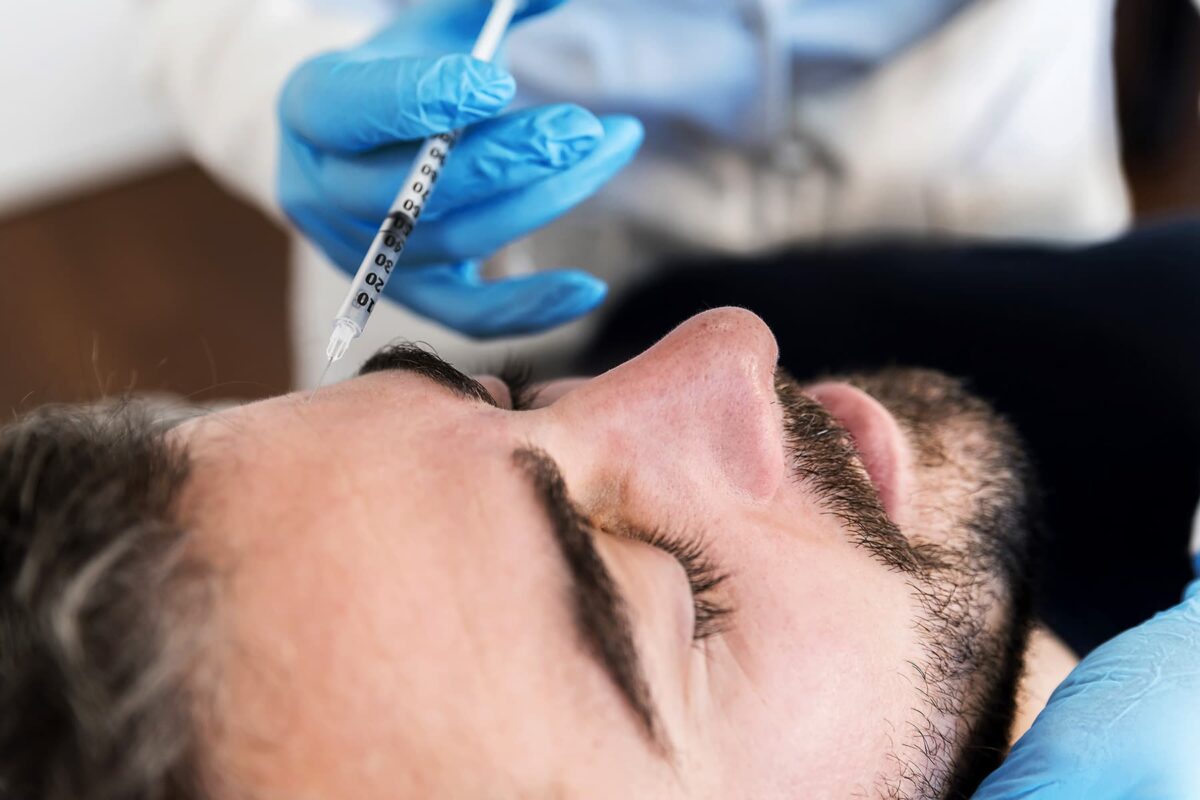
FDA Approves Botox Competitor That Lasts Longer
FRIDAY, Sept. 9, 2022 (HealthDay News) — People wanting to maintain wrinkles at bay will quickly have a brand new choice now that the U.S. Food and Drug Administration has authorised the primary competitor for Botox in a long time.
Daxxify, made by Revance Therapeutics Inc. in Nashville, Tenn., is injected into the face alongside fear traces. It lasts longer than Botox, with about 80% of customers seeing no or delicate facial traces at 4 months after injection. For half of customers, the remedy lasted six months, the corporate mentioned in an announcement.
“Users do not have to go once every three months,” Dr. Balaji Prasad, who covers specialty prescription drugs as an analyst for Barclays Investment Bank, advised the New York Times. “In a world where time is of the essence, having a product with a long duration factor is extremely useful.”
The new drug now enters the $3 billion subject of facial injection medication. It can also be a neuromuscular blocking agent and a botulinum toxin, like Abbvie’s Botox.
“It also opens up the door for what we can do with therapeutics,” mentioned Revance CEO Mark Foley, advised the Times. “If you think of migraines, cervical dystonia [a neurological condition that affects the muscles in the neck and shoulders], overactive bladder, there’s a huge medical opportunity as well.”
The firm has begun testing the drug on these different medical points, Foley mentioned. While the corporate had been attempting to create a product that wanted no needle, it as a substitute found a means to make use of peptide know-how to maintain the product secure. Typically, animal protein or human serum is used.
Botox can also be used for extra than simply wrinkles. It has been an FDA-approved remedy for continual migraines since 2010.
Users of Daxxify within the Revance research included some who skilled unwanted side effects. About 2% of individuals developed a drooping eyelid, whereas about 6% skilled headache, the corporate mentioned.
Toxin-based remedy can carry the potential for different unwanted side effects, resembling common muscle weak spot or respiration difficulties, the FDA cautioned. Daxxify examine members confirmed none of these signs.
Revance had initially hoped for approval of its product in November 2020, however plans had been postponed due to pandemic journey restrictions, the Times reported. An inspection lastly performed in June 2021 discovered issues with the standard management course of and the corporate’s working cell financial institution, which include the drug’s energetic ingredient. Those issues had been resolved, the Times reported.
More data
The National Library of Medicine has extra about botulinum toxin.